August 24, 2021
Life value
STELLAPHARM was born to care and protect patient’s health, to help enhancing their lives and living longer. Your health, for today and for future.
A novel coronavirus, originally identified in Wuhan City, China, was reported to the World Health Organization on 31 December 2019, and the associated disease has subsequently become a worldwide pandemic. An effective antiviral therapeutic has since been intensively sought.
Coronaviruses use an RNA-dependent RNA polymerase (RdRp) for the replication and transcription of their RNA genome. RdRp is an important target for the development of antiviral drugs against coronaviruses. Structures of RdRp have been reported for SARS-CoV-1 and SARS-CoV-2 and provide insights into the mechanisms of RNA-dependent RNA synthesis. The structures also enable mechanistic studies that can rationalize the molecular processes underlying the antiviral activity of compounds targeting RdRp. [2]
Molnupiravir (also known as EIDD-2801/MK-4482) is a prodrug of the active antiviral ribonucleoside analog β-d-N4-hydroxycytidine (NHC; EIDD-1931), which has demonstrated the potential to treat infections caused by multiple RNA viruses, including highly pathogenic coronaviruses and influenza viruses, and encephalitic alphaviruses such as Venezuelan, Eastern, and Western equine encephalitis viruses, in nonclinical models. [1]
Molnupiravir is an orally available antiviral drug candidate currently in phase III trials for the treatment of patients with COVID-19. Molnupiravir increases the frequency of viral RNA mutations and impairs SARS-CoV-2 replication in animal models and in humans. [2]
Molecular echanism of molnupiravir-induced SARS-CoV-2 mutagenesis
Molnupiravir is quickly cleaved in plasma to EIDD-1931, which after distribution into various tissues, is converted to its corresponding 59-triphosphate by host kinases (Figure 1). EIDD-1931 59-triphosphate is the active form of molnupiravir. [1]
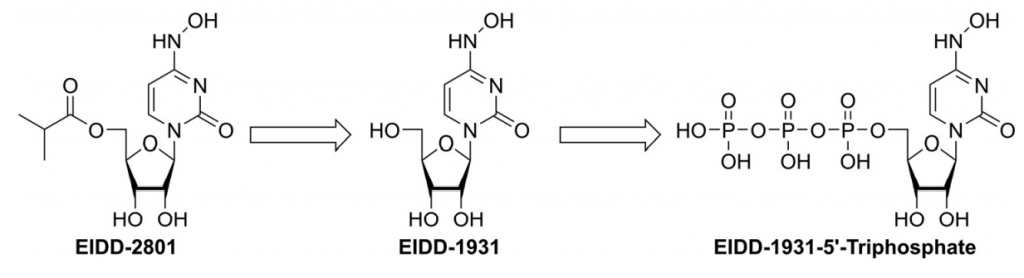
Figure 1. Molnupiravir is rapidly converted in the plasma to EIDD-1931 (NHC), which after distribution into various tissues is converted by host kinases into EIDD-1931 59-triphosphate, the active antiviral agent. (1)
Biochemical assays show that the viral RNA-dependent RNA polymerase (RdRp) uses EIDD-1931-5′-triphosphat as a substrate instead of cytidine triphosphate or uridine triphosphate. When the RdRp uses the resulting RNA as a template, EIDD-1931-5′-triphosphate directs incorporation of either G or A, leading to mutated RNA products (Figure 2). Upon incorporation into nascent chain viral RNA, EIDD-1931-5′-triphosphat induces an antiviral effect via viral error catastrophe, a concept that is predicated on increasing the viral mutation rate beyond a biologically tolerable threshold, resulting in impairment of viral fitness and leading to viral extinction. In addition, structural analysis of RdRp–RNA complexes that contain mutagenesis products shows that EIDD-1931-5′-triphosphat can form stable base pairs with either G or A in the RdRp active center, explaining how the polymerase escapes proofreading and synthesizes mutated RNA. [1], [2]
This two-step mutagenesis mechanism probably applies to various viral polymerases and can explain the broad-spectrum antiviral activity of molnupiravir. [2]
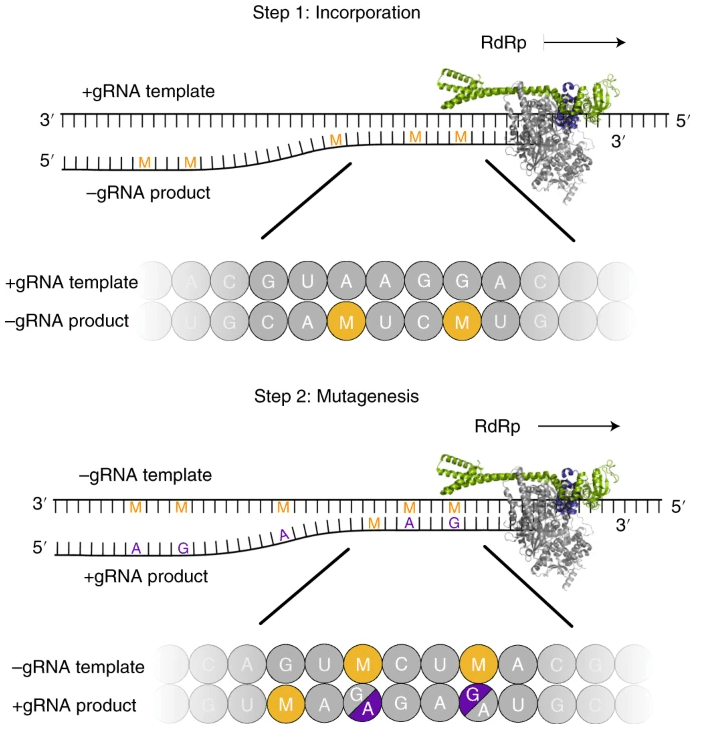
Figure 2. Two-step model of molnupiravir-induced RNA mutagenesis. [2]
(EIDD-1931-5′-triphosphat (M) can be used by the RdRp of SARS-CoV-2 as a substrate instead of cytidine triphosphate (C) or uridine triphosphateor (U). Therefore, in a first step, the RdRp is predicted to frequently incorporate M instead of C or U when it uses the positive-strand genomic RNA (+gRNA) as a template to synthesize negative-strand genomic (−gRNA) and subgenomic RNA (−sgRNA). In a second step, the resulting M-containing RNA can be used as a template for the synthesis of +gRNA or positive-strand subgenomic mRNA (+sgmRNA). The presence of M in the −gRNA then leads to mutations in the positive-strand RNA products, which do not support formation of intact new viruses, as predicted by the ‘error catastrophe’ mode.)
Results of studies on molnupiravir in humans to date
1. Pharmacokinetics
Single and multiple doses of molnupiravir were evaluated in this first-in-human, phase 1, andomized, double-blind, placebo-controlled study in healthy volunteers, which included evaluation of the effect of food on pharmacokinetics.
Molnupiravir is well absorbed after oral administration and absorption is minimally affected by food intake. EIDD-1931 appeared rapidly in plasma, with a median time of maximum observed concentration of 1.00 to 1.75h, and declined with a geometric half-life of approximately 1h, with a slower elimination phase apparent following multiple doses or higher single doses (7.1h at the highest dose tested). Mean maximum observed concentration (Cmax) and area under the plasma concentration versus time curve (AUC) increased in a dose-proportional manner, and there was no accumulation following multiple doses. When administered in a fed state, there was a decrease in the rate of absorption, but no decrease in overall exposure. [1]
2. Tolerability and safety
According to the results of Phase I study, molnupiravir was well tolerated at doses of 50 to 800 mg administered BID for 5.5 days and at single doses up to 1,600 mg. There were no serious or severe adverse events, and there were no trends of increased frequency or severity of adverse events with higher doses of molnupiravir. Fewer than half of the subjects reported an adverse event, the incidence of adverse events was higher following administration of placebo, and 93.3% of adverse events were mild. Adverse effects commonly reported were mild (Grade 1 – 2) and included flu-like and upper respiratory symptoms, headache, myalgia, diarrhoea and nausea which were also consistent with symptomatic COVID-19 disease. There were no clinically significant findings in clinical laboratory, vital signs, or electrocardiography, and there were no clinically significant changes in hematological parameters seen in this study. [1]
Safety analyses from Phase I trial were consistent with those from a Phase I trial of molnupiravir and support ongoing clinical development. Overall, molnupiravir was well tolerated with no increase in treatment-related or serious adverse events compared to participants administered placebo. There were no safety signals or evidence of hematologic, renal, or hepatic toxicity at any dose. [3]
3. Clinical efficacy
Results of a Phase 2a, double-blind, placebo-controlled, randomized, multicenter trial designed to evaluate the safety, tolerability, and antiviral activity of molnupiravir dosed twice-daily for 5 days in the treatment of 202 patients with mild to moderate COVID-19 showed that molnupiravir is highly effective at reducing nasopharyngeal SARS-CoV-2 infectious virus and viral RNA and has a favorable safety and tolerability profile. [3]
Infectious virus was isolated from 43.5% of evaluable nasopharyngeal swabs at baseline. Virus isolation was significantly lower in participants receiving 800 mg molnupiravir (1.9%) versus placebo (16.7%) at Day 3 (p = 0.02). At Day 5, virus was not isolated from any participants receiving 400 or 800 mg molnupiravir, versus 11.1% of those receiving placebo (p = 0.03). Time to viral RNA clearance was decreased and a greater proportion overall achieved clearance in participants administered 800 mg molnupiravir versus placebo (p = 0.01). Molnupiravir was generally well tolerated, with similar numbers of adverse events across all groups. [3]
Only 22.8% of participants had evidence of a humoral immune response at baseline. However, by Day 28, 92.1% were antibody positive, demonstrating that early treatment with molnupiravir had an antiviral effect without inhibiting the development of a humoral immune response. [3]
Ongoing studies
Merck, in collaboration with Ridgeback Biotherapeutics, is conducting an ongoing randomized placebo-controlled phase 2/3 clinical trial of molnupiravir in non-hospitalized patients with confirmed COVID-19 at more than 100 sites worldwide. [7]
The Phase 3 portion of the global MOVe-OUT (or MK-4482-002) trial studying molnupiravir in non-hospitalized adult patients (18 years or older) with laboratory-confirmed COVID-19 and at least one risk factor associated with poor disease outcomes (such as fever, cough, or loss of taste or smell) is underway (Figure 3). In addition, Merck plans to initiate a clinical program to evaluate molnupiravir for post-exposure prophylaxis in the second half of 2021. [4], [5]
Figure 3. Merck’s COVID-19 Clinical Trial. [5]
Expanding global access to molnupiravir
According to Merck president Rob Davis, in addition the agreement with the US government about supplying 1.7 million courses of molnupiravir to US, Merck is actively engaged in numerous efforts to make molnupiravir available globally to fulfil Merck’s commitment to widespread access. The company is planning to apply for emergency use or approval in other countries outside the US. In April this year, Merck signed voluntary licensing agreements with five Indian generic drug manufacturers to expand access to molnupiravir. The company also signed non-exclusive voluntary licensing agreements with additional generic manufactures to offer the drug in 104 low- and middle-income countries. [6]
One of these generics manufacturers, Hetero, has announced that it has accumulated positive data from an open label clinical study of molnupiravir and has submitted these data to the Drug Controller General of India (DCGI). Merck is encouraged by these promising data and looks forward to continuing to work with Hetero and other generics manufacturers that Merck has agreements with as they conduct studies of generic versions of investigational molnupiravir. [7]
Source:
[1] Painter et al., Human safety, tolerability, and pharmacokinetics of Molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2, Antimicrob Agents Chemother, Volume 65, Issue 5, May 2021.
[2] Florian Kabinger et al., Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nature Structural & Molecular Biology, August 2021.
[3] William Fischer et al., Molnupiravir, an Oral Antiviral Treatment for COVID-19,MedRxiv, June 2021.
[4] Interim results from Phase 2/3 studies of molnupiravir, an investigational oral antiviral therapeutic for mild to moderate covid-19, presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Merck’s news release, July 2021.
[5] Merck’s COVID-19 Clinical Trial: https://merckcovidresearch.com
[6] https://www.pharmaceutical-business-review.com/news/merck-1-7-million-courses-molnupiravir-us/
[7] Merck Statement on Clinical Data for Molnupiravir Generated by Hetero in India, Merck’s news release, July 2021.
Stellapharm is one of leading generics pharmaceutical companies and strong producer of anti-viral drugs in Vietnam. The company established in Vietnam in 2000; and focuses on both prescription drugs and non-prescription especially in cardiovascular diseases, antiviral drugs, anti-diabetics drugs, etc. and our products are now used by millions of patients in more than 50 countries worldwide.
The company is globally recognized for its quality through our facilities have been audited and approved by stringent authority like EMA, PMDA, Taiwan GMP, local WHO and others.
Additional information for this article: Stellapharm J.V. Co., Ltd. – Branch 1
A: 40 Tu Do Avenue, Vietnam – Singapore Industrial Park, An Phu Ward, Thuan An City, Binh Duong Province, Vietnam
T: +84 274 376 7470 | F: +84 274 376 7469 | E: info@stellapharm.com | W: www.stellapharm.com
[Updated to July 2021] The results of the trial demonstrate the safety, tolerability, and antiviral efficacy of molnupiravir to reduce replication of SARS-CoV-2 and accelerate clearance of infectious virus and support ongoing trials of molnupiravir to prevent progression of COVID-19 and eliminate onward transmission of SARS-CoV-2. After more than one year into the coronavirus disease
On February 21, 2022, Stellapharm officially signed a contract with MPP (Medicines Patent Pool), a UNITED NATIONS-backed public health organization to become the only manufacturer in Vietnam allowed to produce a generic version of Pfizer’s oral COVID-19 treatment, nirmatrelvir, which will be co-packaged with ritonavir, under the voluntary licensing agreement between Pfizer and the MPP.