March 05, 2025
Life value
STELLAPHARM was born to care and protect patient’s health, to help enhancing their lives and living longer. Your health, for today and for future.
The trade deal between Vietnam and the EU has been making significant impacts on the home pharma market, driven by tariff reductions, marketing simplifications, and clearer business rights.
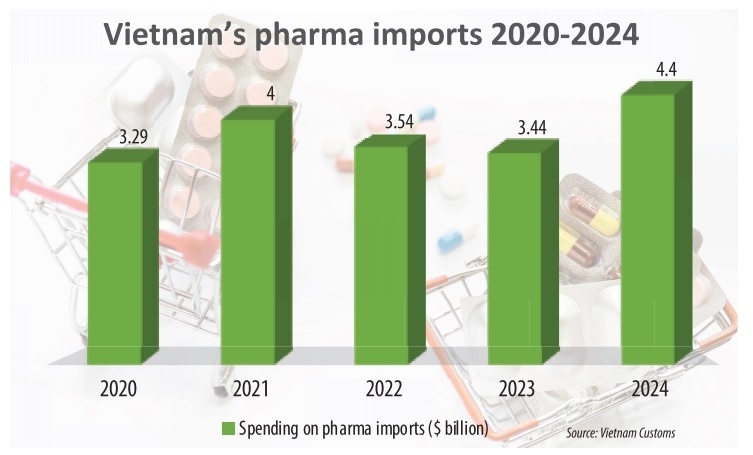
According to the latest statistics from Vietnam Customs, pharmaceutical imports totalled nearly $4.4 billion last year, up 27.9 per cent from 2023, of which $2.5 billion came from the European Union.
“One of the most notable aspects of the EU-Vietnam Free Trade Agreement (EVFTA) for the pharmaceutical industry is the reduction of tariffs on medicinal products and related materials. This has made significant changes in the market and facilitated imports from the EU since August 2020 when the deal took effect,” said industry expert Hai Ngo.
One month later, pharmaceutical imports from the EU increased by 19.1 per cent compared to the same period of the previous year, surpassing the annual import growth rate from the EU five years ago, which was 15.4 per cent.
The major markets supplying pharmaceuticals to Vietnam in 2024 was led by France, reaching $572.46 million and an increase of 31.4 per cent on-year, accounting for 13 per cent of the country’s total pharma import turnover. The runners-up include Germany at $404.8 million, up 26.8 per cent on-year, and Italy, up 57.6 per cent on-year. Belgium, Ireland, Austria, Spain, and Sweden also saw double-digit growth.
The EVFTA requires member states to commit to higher standards than those they have already committed to the World Trade Organization on the same issue. Vietnam commits to eliminating tariffs on approximately 71 per cent of pharmaceutical-related tariff lines upon the agreement’s implementation, with the remaining tariffs being phased out over a maximum period of 10 years.
Certain drug categories have been benefiting from faster tax reductions. For example, HS3003, which includes a variety of pharma products, has the quickest tariff reduction schedule. Six tax lines under this group will see immediate tariff reductions to zero.
Groups like HS3004, which includes a broader range of items, will experience a longer tariff protection period, with 26 tax lines seeing reductions over time. Meanwhile, the HS3005 group, which includes a smaller range of drugs, will undergo an eight-year tariff reduction period.
Together with the tariff reduction, simplification of the marketing authorisation (MA) process and clearer business rights have been leveraging multinational corporations to increase pharma exports to Vietnam.
The country has committed under the EVFTA to align its MA procedures with international standards, aiming for greater transparency and efficiency. One of the key developments in this regard is the Ministry of Health’s (MoH) issuance of rules that simplify the renewal process by reducing the amount of required documentation.
Previously, related companies needed to submit seven documents to renew the MA for their products. With the new regulation, domestic drugs require only two documents, and imported drugs require three. Additionally, the new Pharmaceutical Law also includes provisions aimed at streamlining the renewal of the MA administrative procedures.
It is expected that after the amended Pharmaceutical Law’s provisions on MA renewal procedures take effect in July, the process of extending the validity of certificates will see significant improvements, which will facilitate businesses’ participation and enhance the management and administration efforts of the MoH and relevant authorities.
The EVFTA also facilitates greater foreign investment in Vietnam’s market. The agreement specifically addresses the rights of foreign-invested enterprises (FIEs) to import pharma products into the country. In addition, the 2024 amendment to the law provides clear provisions allowing them to engage in activities such as contract manufacturing, technology transfer, and transportation of products to wholesalers’ warehouses.
“These improvements create a more enabling environment and new opportunities for the EU’s pharma companies, as they can take advantage of lower import tariffs to increase their competitiveness in the Vietnamese market. Recent moves by multinationals prove the impacts,” Hai Ngo added.
Such groups include AstraZeneca Vietnam, which has committed to investing approximately $220 million in the country over the past few years.
Elsewhere, Sanofi views the EVFTA as a significant step forward for pharma imports in Vietnam. It has also made improvements and restructured various aspects, including transportation, storage, quality management, and business operations.
Similarly, Danish-led Novo Nordisk Vietnam achieved its first shipment of products from Denmark in July 2024 as a fully recognised legal foreign entity in the country. With the recognition, Novo Nordisk is now fully recognised by the government as its own legal entity that can import its own medicines to Vietnam, to be sold on to the local distributor.
Other experts, however, said the EVFTA still offers challenges in terms of meeting global standards.
“With the tariff reduction strategy, Vietnam aims to build a more open and efficient pharma market. However, its commitments to eliminate or reduce tariffs have not yet had a significant impact,” said healthcare analyst Duyen Nguyen. “Vietnam will need to continue refining its legal and regulatory framework to fully align with its commitments under the EVFTA, ensuring a thriving pharmaceutical industry.”
Source: Vietnam Investment Review
About STELLAPHARM
Stellapharm J.V. Co., Ltd. is currently known as one of leading generics pharmaceutical companies and strong manufacturers in Vietnam. Established in 2000, Stellapharm was built on the foundation of a manufacturing factory in Vietnam and formed a joint venture with a partner from Germany. We focus on both prescription drugs and non-prescription especially in cardiovascular diseases, anti-diabetics drugs, etc. Products of Stellapharm are now used by millions of patients in more than 50 countries worldwide.
The company is globally recognized for its quality through our facilities have been audited and approved by stringent authority like EMA, PMDA, Taiwan GMP, local WHO and others.
Additional information for this article: Stellapharm J.V. Co., Ltd. – Branch 1
A: 40 Tu Do Avenue, Vietnam – Singapore Industrial Park, An Phu Ward, Thuan An City, Binh Duong Province, Vietnam
T: +84 274 376 7470 | F: +84 274 376 7469 | E: info@stellapharm.com | W: www.stellapharm.com
After more than 5 years of implementation, the Vietnam – EU Free Trade Agreement (EVFTA) has had a strong impact on the Vietnamese economy in general and the pharmaceutical market in particular. EVFTA takes effect from August 1, 2020. The emergence of EVFTA increases the competitiveness between Vietnamese pharmaceutical enterprises and enterprises in the same
With a population of more than 100 million and a total drug consumption of more than 8 billion USD in 2023, the Vietnamese pharmaceutical market is quite attractive and has a lot of potential for production and business activities. The Ministry of Health said that 2024 is a key year, with the review and amendment
Only 42 new drugs out of 460 types circulated globally are available in Vietnam, due to long licensing procedures and time, affecting patients. On January 21, a representative of the Ministry of Health said that generic drug prices in Vietnam are low compared to countries in the ASEAN region (for most main treatment groups). While
With the development of the global economy, the growth of the total population and the aging of society, the demand for drugs is increasing, and the market size of the global pharmaceutical industry will maintain growth. stable.In 2023, the total global pharmaceutical market is estimated to be approximately US$1,6 trillion. This is an increase of
The top 10 businesses consist of Binh Thuan Plastic Group JSC, HD Securities Corporation, Orient Securities Corporation, Imedia Technology and Services JSC, SOL E&C Construction Investment JSC, Vietnam Vitadairy Milk JSC, Taseco Land Investment JSC, Stellapharm J.V. Co. Ltd., CNC Technology Solutions JSC, and Bee Logistics Corporation. The top 10 in the 2024 list